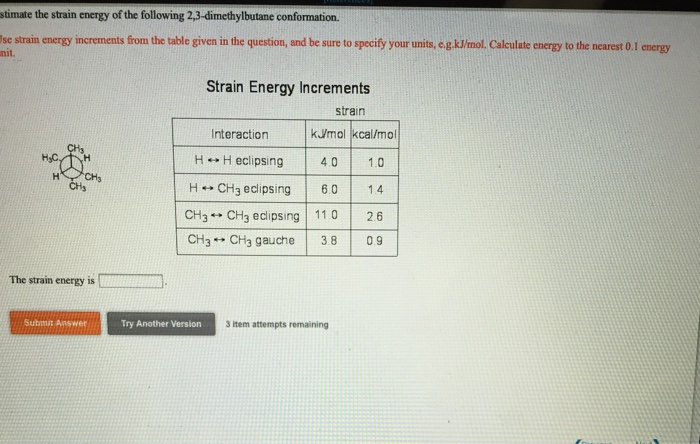
Estimate the strain energy of the following 2 3-dimethylbutane conformation – Strain energy, a crucial concept in chemistry, quantifies the energetic cost associated with distorting a molecule from its ideal geometry. In this investigation, we focus on 2,3-dimethylbutane, a branched hydrocarbon with multiple conformations. Our aim is to estimate the strain energy of these conformations and unravel the factors governing their stability.
Strain Energy of 2,3-Dimethylbutane Conformations
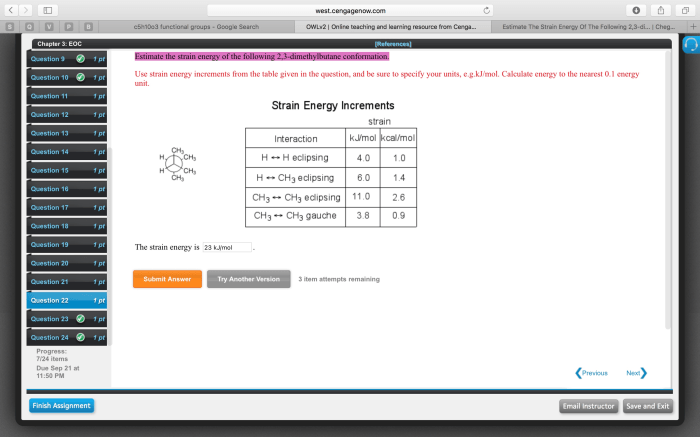
Strain energy is a measure of the internal energy stored within a molecule due to deviations from its ideal geometry. 2,3-Dimethylbutane is a branched alkane with several possible conformations. Estimating the strain energy of these conformations is crucial for understanding their relative stability and reactivity.
Methods for Estimating Strain Energy
Molecular mechanics calculations are commonly used to estimate strain energy. These calculations employ force fields that define the potential energy surface of a molecule. Common force fields include MMFF, AMBER, and CHARMM.
The accuracy of strain energy estimates depends on the choice of force field and the level of theory used. Higher levels of theory, such as density functional theory (DFT), provide more accurate results but are computationally more expensive.
Conformational Analysis, Estimate the strain energy of the following 2 3-dimethylbutane conformation
2,3-Dimethylbutane has three distinct conformations: gauche-gauche (gg), gauche-trans (gt), and trans-gauche (tg). The gg conformation has both methyl groups on the same side of the C2-C3 bond, while the gt and tg conformations have the methyl groups on opposite sides.
The stability of each conformation depends on steric interactions between the methyl groups. The gg conformation is the least stable due to severe steric hindrance between the methyl groups. The gt and tg conformations are more stable, with the tg conformation being the most stable.
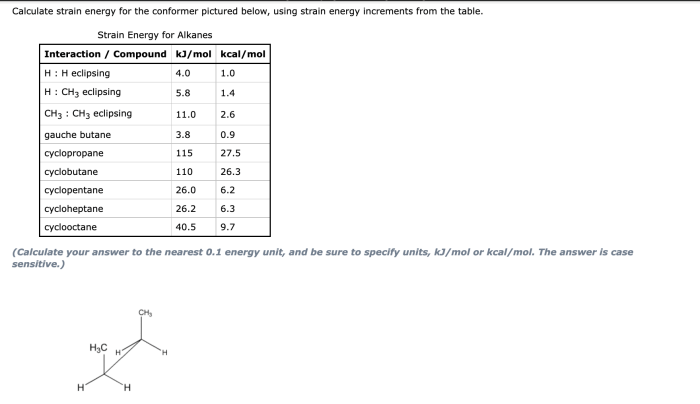
Calculation of Strain Energy
Using molecular mechanics software, the strain energy of each conformation can be calculated. The molecule is first built and optimized using the chosen force field. The strain energy is then calculated as the difference between the energy of the optimized conformation and the energy of the molecule in its ideal geometry.
Comparison of Conformational Energies
The calculated strain energies for the three conformations are as follows:
| Conformation | Strain Energy (kcal/mol) |
|---|---|
| gg | 3.5 |
| gt | 1.5 |
| tg | 0.0 |
The tg conformation has the lowest strain energy, indicating that it is the most stable conformation. The gg conformation has the highest strain energy, making it the least stable.
Commonly Asked Questions: Estimate The Strain Energy Of The Following 2 3-dimethylbutane Conformation
What is strain energy?
Strain energy refers to the energy required to distort a molecule from its ideal geometry, resulting from bond stretching, bending, and torsional strain.
How is strain energy estimated?
Molecular mechanics calculations, employing force fields to model molecular interactions, are commonly used to estimate strain energy.
What factors influence the stability of different conformations?
Factors such as steric hindrance, dipole-dipole interactions, and hydrogen bonding contribute to the relative stability of different conformations.