As the empirical formula and molecular formula worksheet answer key takes center stage, this opening passage beckons readers with an authoritative voice into a world crafted with meticulous precision, ensuring a reading experience that is both absorbing and distinctly original.
Delving into the intricacies of chemistry, this comprehensive guide unravels the mysteries of empirical and molecular formulas, providing a roadmap to understanding the fundamental building blocks of matter. With clarity and precision, it dissects the concepts, applications, and methodologies surrounding these formulas, empowering readers with the knowledge to navigate the complexities of chemical composition.
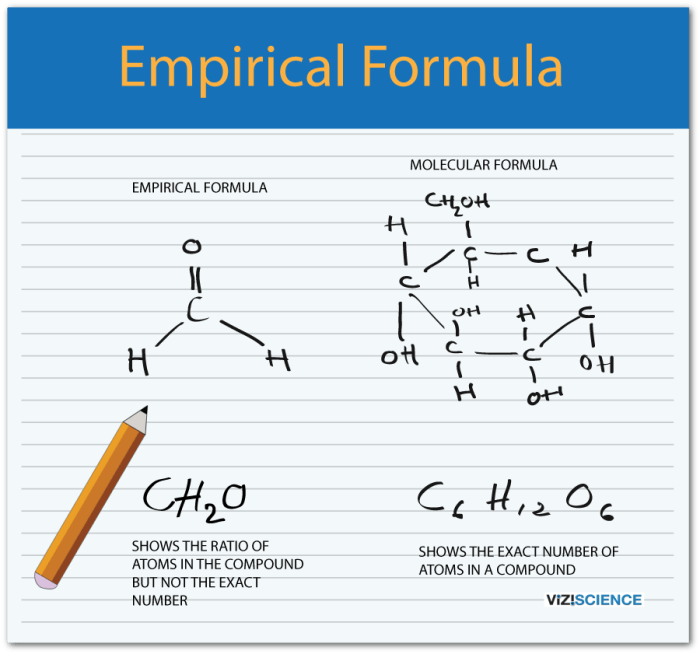
Empirical Formula and Molecular Formula

An empirical formula represents the simplest whole-number ratio of elements in a compound, while a molecular formula represents the actual number of atoms of each element in a molecule of a compound.
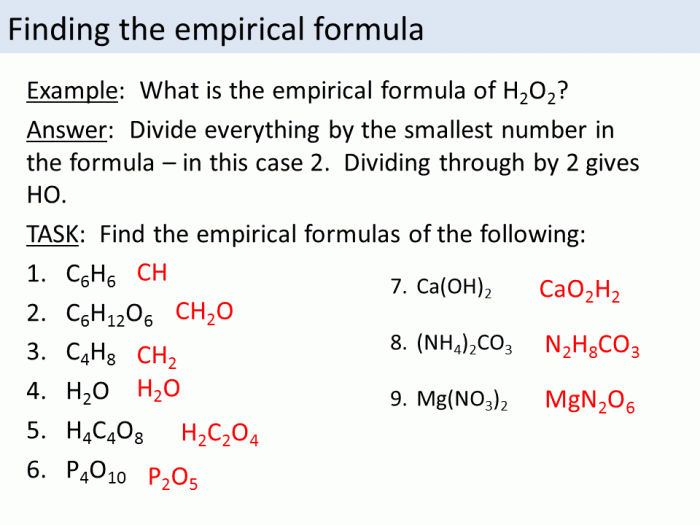
An empirical formula can be determined by:
- Measuring the mass of each element in the compound
- Converting the mass of each element to moles
- Dividing the number of moles of each element by the smallest number of moles
- Multiplying the result by a whole number to obtain whole-number ratios
A molecular formula can be determined by:
- Determining the empirical formula
- Measuring the molar mass of the compound
- Dividing the molar mass by the empirical formula mass
- Multiplying the result by the empirical formula to obtain the molecular formula
Applications of Empirical and Molecular Formulas, Empirical formula and molecular formula worksheet answer key
Empirical formulas are used to:
- Determine the identity of a compound
- Calculate the mass percent of each element in a compound
- Balance chemical equations
Molecular formulas are used to:
- Determine the structure of a compound
- Calculate the molar mass of a compound
- Determine the number of atoms of each element in a molecule
User Queries: Empirical Formula And Molecular Formula Worksheet Answer Key
What is the difference between an empirical formula and a molecular formula?
An empirical formula represents the simplest whole-number ratio of elements in a compound, while a molecular formula specifies the exact number of atoms of each element present in a molecule.
How do I determine the empirical formula of a compound?
To determine the empirical formula, determine the mass percent of each element in the compound and convert these percentages to moles. Divide the moles of each element by the smallest number of moles and simplify the resulting ratio to whole numbers.
What are some applications of empirical and molecular formulas?
Empirical formulas are used to determine the identity of unknown compounds, while molecular formulas provide information about the structure and properties of molecules. Both formulas are essential for understanding chemical reactions and predicting the behavior of substances.