Melting point of 4 tert butylcyclohexanone – The melting point of 4-tert-butylcyclohexanone, an important chemical compound, is a fundamental property with wide-ranging applications. This article delves into the molecular structure, properties, methods for determining the melting point, factors affecting it, and its significance in various fields.
4-tert-Butylcyclohexanone is a cyclic ketone with a unique molecular structure that influences its physical and chemical properties, including its melting point. Understanding the relationship between structure and properties is crucial for predicting and optimizing its behavior in different applications.
Melting Point of 4-tert-Butylcyclohexanone

4-tert-Butylcyclohexanone is a cyclic ketone with a molecular formula of C 10H 18O. It is a colorless liquid with a characteristic odor. The melting point of 4-tert-butylcyclohexanone is an important physical property that can be used for its identification and characterization.
Chemical Structure and Properties, Melting point of 4 tert butylcyclohexanone
The molecular structure of 4-tert-butylcyclohexanone consists of a cyclohexane ring with a tert-butyl group attached to the fourth carbon atom. The tert-butyl group is a bulky substituent that influences the physical and chemical properties of the compound.
| Property | Value |
|---|---|
| Molecular formula | C10H18O |
| Molecular weight | 154.25 g/mol |
| Density | 0.893 g/mL (25 °C) |
| Boiling point | 205-207 °C |
| Melting point | 26-28 °C |
| Solubility in water | Insoluble |
| Solubility in organic solvents | Soluble |
The tert-butyl group imparts steric hindrance around the carbonyl group, which affects the reactivity of the compound. 4-tert-Butylcyclohexanone is less reactive than other cyclohexanones due to the steric hindrance.
Melting Point Determination
The melting point of 4-tert-butylcyclohexanone can be determined using various methods, including:
- Capillary method
- Hot stage microscopy
- Differential scanning calorimetry (DSC)
The capillary method is a simple and widely used technique. It involves filling a capillary tube with the sample and heating it gradually while observing the temperature at which the sample melts.
Factors Affecting Melting Point
The melting point of 4-tert-butylcyclohexanone can be affected by several factors, including:
- Impurities: Impurities can lower the melting point of a compound.
- Crystal size: Smaller crystals have a lower melting point than larger crystals.
- Heating rate: A faster heating rate can result in a higher melting point.
It is important to control these factors when determining the melting point of 4-tert-butylcyclohexanone to ensure accurate results.
Applications of Melting Point Data
Melting point data is useful for various applications, including:
- Compound identification: Melting point can be used to identify compounds by comparing the observed melting point with known values.
- Purity assessment: The melting point of a pure compound is typically sharp and well-defined. The presence of impurities can broaden the melting point range.
- Quality control: Melting point can be used as a quality control parameter to ensure the purity and consistency of products.
Melting point data is also used in various industries, such as pharmaceuticals, chemicals, and food.
Related Compounds and Melting Points
The melting points of related compounds can provide insights into the structure-property relationship. Some related compounds to 4-tert-butylcyclohexanone and their melting points include:
- Cyclohexanone: -63.5 °C
- 4-Methylcyclohexanone: -42.6 °C
- 4-Isopropylcyclohexanone: 23-25 °C
The melting point of 4-tert-butylcyclohexanone is higher than that of cyclohexanone and 4-methylcyclohexanone due to the presence of the bulky tert-butyl group. The melting point of 4-isopropylcyclohexanone is similar to that of 4-tert-butylcyclohexanone, which suggests that the size of the alkyl substituent has a significant effect on the melting point.
Key Questions Answered: Melting Point Of 4 Tert Butylcyclohexanone
What is the molecular structure of 4-tert-butylcyclohexanone?
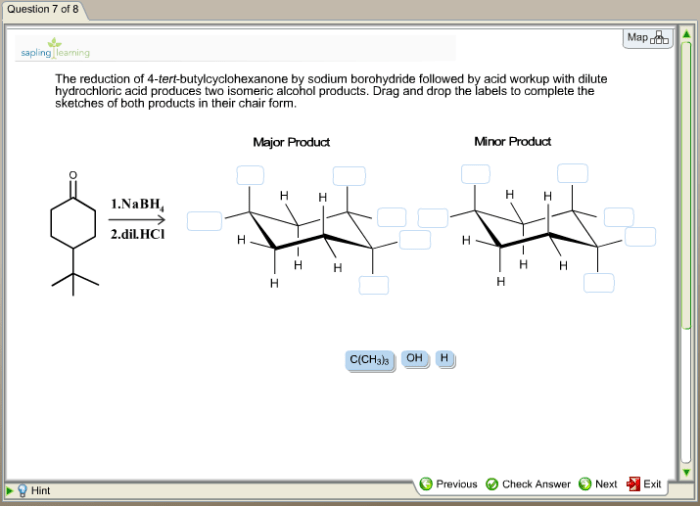
4-tert-Butylcyclohexanone has a cyclic structure with a six-membered carbon ring and a ketone group (C=O) attached to the fourth carbon atom. The tert-butyl group (C(CH3)3) is attached to the same carbon atom as the ketone group.
How is the melting point of 4-tert-butylcyclohexanone determined?
The melting point can be determined using various methods, including the capillary method, the Kofler hot-stage method, and differential scanning calorimetry (DSC). Each method has its own advantages and limitations in terms of accuracy and sensitivity.
What factors can affect the melting point of 4-tert-butylcyclohexanone?
Impurities, crystal size, and heating rate can all influence the observed melting point. Impurities can lower the melting point, while larger crystals and faster heating rates can lead to higher melting points.