Embark on a scientific expedition with the phet kinetic molecular theory answer key, an invaluable resource that unlocks the secrets of gas behavior. This key provides a comprehensive understanding of the fundamental principles governing gases, empowering students and researchers alike to delve into the fascinating world of molecular motion and its impact on macroscopic properties.
The phet kinetic molecular theory simulation serves as a virtual laboratory, allowing users to manipulate gas properties, observe particle interactions, and witness the interplay between temperature, volume, and pressure. Through interactive experiments and data analysis, learners gain hands-on experience in exploring the behavior of gases, making abstract concepts tangible and accessible.
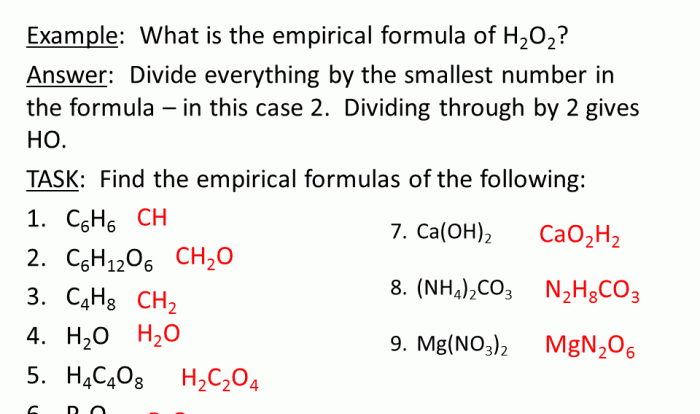
1. Phet Kinetic Molecular Theory Introduction
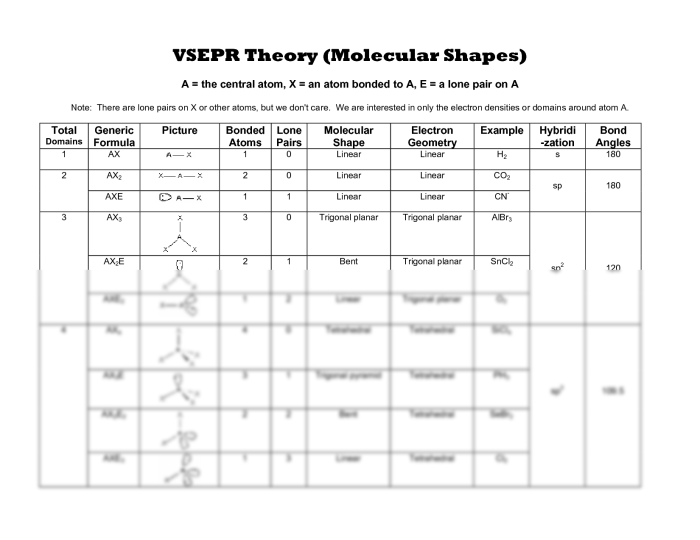
The Phet Kinetic Molecular Theory simulation is an interactive tool designed to help students visualize and understand the behavior of gases at the molecular level. It demonstrates the fundamental principles of kinetic molecular theory, which explains the properties of gases based on the motion and interactions of their constituent particles.
Key concepts and principles illustrated by the simulation include:
- Particles in a gas are in constant random motion.
- The average kinetic energy of particles is proportional to the temperature of the gas.
- Collisions between particles are elastic, meaning that total kinetic energy is conserved.
- The pressure exerted by a gas is due to the collisions of its particles with the walls of the container.
2. Simulation Interface and Features
The Phet Kinetic Molecular Theory simulation interface consists of a central display area and a set of controls on the right side. The display area shows a container filled with gas particles, which can be viewed in either 2D or 3D.
The controls allow users to manipulate the following parameters:
- Temperature
- Volume
- Number of particles
- Particle size
- Collision frequency
Users can also choose to display additional information, such as particle velocities, kinetic energies, and pressure.
3. Gas Properties and Behavior

The Phet Kinetic Molecular Theory simulation allows users to observe the properties of gases and how they change under different conditions. By manipulating the simulation parameters, users can see how temperature, pressure, and volume affect the behavior of gas particles.
For example, increasing the temperature of a gas causes the particles to move faster and collide more frequently. This leads to an increase in pressure and volume. Conversely, decreasing the temperature causes the particles to slow down and collide less frequently, which leads to a decrease in pressure and volume.
4. Particle Motion and Collisions
The Phet Kinetic Molecular Theory simulation allows users to visualize the motion of gas particles and the collisions that occur between them. Particles are represented as small spheres that move in random directions. When two particles collide, they bounce off each other without losing any energy.
The simulation also allows users to control the frequency of collisions. By increasing the collision frequency, users can see how the behavior of the gas changes. For example, increasing the collision frequency causes the particles to move more slowly and collide more frequently, which leads to an increase in pressure and a decrease in volume.
5. Temperature and Kinetic Energy: Phet Kinetic Molecular Theory Answer Key
The Phet Kinetic Molecular Theory simulation demonstrates the relationship between temperature and kinetic energy. Temperature is a measure of the average kinetic energy of the particles in a gas. As the temperature of a gas increases, the average kinetic energy of the particles also increases.
The simulation allows users to visualize the distribution of kinetic energies among the particles in a gas. By displaying a histogram of particle kinetic energies, users can see how the distribution changes as the temperature of the gas changes.
Detailed FAQs
What is the purpose of the phet kinetic molecular theory simulation?
The phet kinetic molecular theory simulation is designed to provide an interactive and engaging platform for students and researchers to explore the behavior of gases and gain a deeper understanding of the fundamental principles governing their properties and interactions.
How does the simulation represent particle motion?
The simulation represents particle motion using colored dots that move around the simulation space. The speed and direction of the dots represent the velocity and kinetic energy of the particles.
What is the relationship between temperature and kinetic energy in the simulation?
In the simulation, temperature is directly proportional to the average kinetic energy of the particles. As the temperature increases, the particles move faster and have higher kinetic energy.