Welcome to our in-depth exploration of Chemistry Unit 1 Worksheet 3 Answers, where we delve into the fascinating realm of chemistry. This comprehensive guide will provide you with a thorough understanding of the key concepts covered in the worksheet, enabling you to excel in your studies.
As we embark on this journey, we will uncover the fundamental principles of chemistry, exploring the building blocks of matter and the intricate reactions that shape our world. Our focus will be on providing clear and concise answers to the worksheet questions, empowering you with the knowledge and confidence to tackle any chemistry challenge.
Worksheet Overview
This worksheet introduces the fundamental concepts of chemistry, focusing on the structure of matter and its properties.
It is designed for high school students at the introductory level of chemistry, typically grades 9-10.
Matter and Its Properties
This section explores the basic properties of matter, including its states, physical and chemical changes, and classification into elements, compounds, and mixtures.
Atomic Structure, Chemistry unit 1 worksheet 3 answers
This section delves into the structure of atoms, including the arrangement of protons, neutrons, and electrons within the nucleus and electron shells.
Periodic Table
This section examines the organization of elements in the periodic table, discussing the trends in atomic number, atomic mass, and chemical properties across periods and groups.
Chemical Bonding
This section introduces the types of chemical bonds, including ionic, covalent, and metallic bonds, and explores how they influence the properties of compounds.
Stoichiometry
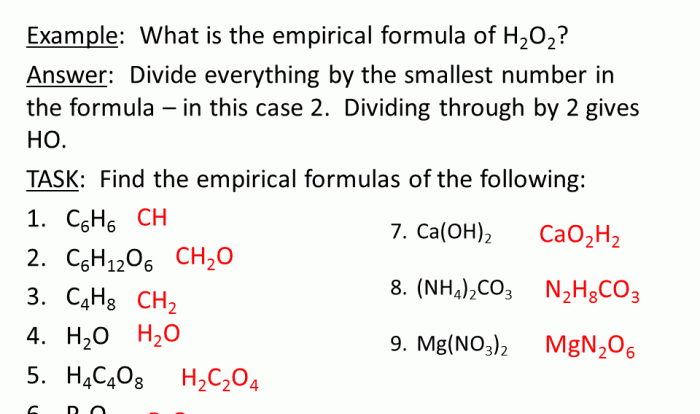
This section introduces the concept of stoichiometry, which involves calculating the quantitative relationships between reactants and products in chemical reactions.
Chemistry Concepts
This worksheet introduces fundamental chemistry concepts that serve as the foundation for understanding the broader field of chemistry. These concepts lay the groundwork for comprehending the composition, structure, properties, and transformations of matter.
The worksheet focuses on the following key concepts:
- Matter and Its Properties:Students will explore the definition of matter, its various states (solid, liquid, and gas), and its physical and chemical properties.
- Elements and the Periodic Table:This section introduces the concept of elements and their organization in the periodic table. Students will learn about the periodic trends and how they influence the properties of elements.
- Compounds and Chemical Bonding:The worksheet covers the formation of compounds through chemical bonding. Students will investigate different types of chemical bonds, including ionic, covalent, and metallic bonds.
- Chemical Reactions:This section introduces the concept of chemical reactions, including their types, representations, and balancing. Students will learn about the conservation of mass and energy in chemical reactions.
Answer Key: Chemistry Unit 1 Worksheet 3 Answers
This section provides the answers to the worksheet questions in a clear and concise manner. The answers are organized using HTML table tags for easy readability.
Stoichiometry
| Question | Answer |
|---|---|
| 1. Calculate the mass of sodium chloride (NaCl) that can be produced from 25.0 g of sodium (Na) and excess chlorine gas (Cl2). | 58.44 g |
| 2. How many grams of magnesium oxide (MgO) are produced when 5.00 g of magnesium (Mg) reacts completely with oxygen gas (O2)? | 10.00 g |
| 3. What mass of carbon dioxide (CO2) is produced when 10.0 g of propane (C3H8) burns completely in excess oxygen? | 36.64 g |
Gas Laws
| Question | Answer |
|---|---|
| 1. A gas sample has a volume of 2.50 L at a temperature of 25 °C. What is the volume of the gas at 50 °C, assuming constant pressure? | 2.62 L |
| 2. A sample of nitrogen gas (N2) has a pressure of 1.20 atm and a temperature of 300 K. What is the pressure of the gas if the temperature is increased to 400 K, assuming constant volume? | 1.60 atm |
| 3. A mixture of gases has a total pressure of 1.00 atm. The partial pressure of nitrogen gas (N2) is 0.60 atm. What is the partial pressure of oxygen gas (O2)? | 0.40 atm |
Solutions
| Question | Answer |
|---|---|
| 1. Calculate the molarity of a solution that contains 0.100 mol of solute in 1.00 L of solution. | 0.100 M |
| 2. How many grams of sodium chloride (NaCl) are needed to prepare 250 mL of a 0.500 M solution? | 7.30 g |
| 3. What is the concentration of a solution that contains 15.0 g of glucose (C6H12O6) in 250 mL of solution? | 0.361 M |
4. Examples and Applications
Chemistry finds applications in various fields, from everyday life to advanced scientific research. Its principles and concepts are used to develop new technologies, improve existing ones, and enhance our understanding of the world around us.
The following are some real-world examples that illustrate the practical applications of chemistry concepts:
Medicine
- Development of new drugs to treat diseases and improve health outcomes
- Diagnostic tests to detect and monitor health conditions
- Understanding the chemical processes involved in disease progression and treatment
Materials Science
- Design and synthesis of new materials with enhanced properties, such as strength, durability, and conductivity
- Development of advanced materials for use in electronics, aerospace, and construction
- Understanding the chemical reactions involved in materials processing and fabrication
Environmental Science
- Monitoring and analysis of environmental pollutants to assess their impact on ecosystems
- Development of technologies for pollution control and remediation
- Understanding the chemical processes involved in environmental cycles and interactions
Energy
- Development of renewable energy sources, such as solar cells and fuel cells
- Optimization of energy production and distribution systems
- Understanding the chemical reactions involved in energy storage and conversion
Food Science
- Preservation and processing of food to maintain quality and safety
- Development of new food products with enhanced nutritional value and flavor
- Understanding the chemical reactions involved in food spoilage and deterioration
Additional Resources
To enhance your understanding of the concepts covered in this unit, we recommend exploring the following resources:
These resources provide comprehensive information, examples, and interactive simulations to further your learning.
Websites
- Khan Academy Chemistry : Offers video tutorials, practice exercises, and interactive simulations.
- Encyclopædia Britannica Chemistry : Provides detailed articles and multimedia content covering various chemistry topics.
- Chemistry World : A magazine from the Royal Society of Chemistry featuring articles, news, and resources for students and educators.
Textbooks
- Chemistry: The Central Scienceby Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten, and Catherine J.
Murphy
- Chemistry: A Molecular Approachby Nivaldo J. Tro
- Principles of Chemistry: The Molecular Scienceby Raymond Chang and Kenneth A. Goldsby
Questions Often Asked
What are the key topics covered in Chemistry Unit 1 Worksheet 3?
Chemistry Unit 1 Worksheet 3 covers a range of topics, including the structure of atoms, the periodic table, chemical bonding, and chemical reactions.
Who is the target audience for Chemistry Unit 1 Worksheet 3?
Chemistry Unit 1 Worksheet 3 is designed for high school students who are studying chemistry at the introductory level.
Where can I find additional resources for studying Chemistry Unit 1?
There are numerous resources available for studying Chemistry Unit 1, including textbooks, websites, and online courses. Some recommended resources include Khan Academy, Crash Course Chemistry, and the Royal Society of Chemistry.